Introduction:
The name Catalyst originally comes from the Greek word Katalysis (meaning decomposition). Since the end of 1992, a catalyst has been necessary to meet environmental requirements. Exhaust gases contain harmful substances: CO (carbon monoxide), NOx (nitrogen oxide), and CH (unburned hydrocarbon). These substances are (oxidized) into non-harmful substances. Hence the name Oxidation Catalyst.
In chemistry, a catalyst is understood to be a substance that induces and accelerates or slows down a chemical reaction without undergoing any change itself.

Operation of Three-way / Oxidation Catalyst:
A catalyst is not a filter but can be seen as a conversion element in which noble metals, such as platinum, rhodium, or palladium, are applied. When the exhaust gases come into contact with this, a very rapid chemical reaction occurs. The molecules of the harmful gases are decomposed and combined with other molecules, resulting in a non-harmful gas. The catalyst is able to purify the exhaust gases by 90%. However, this comes at the expense of higher fuel consumption and lower power. This is because it creates a certain air resistance in the exhaust path.
Substances in the exhaust gases:
- CO2: Carbon dioxide (harmful at high concentrations for the environment, humans, and animals)
- CO: Carbon monoxide (incompletely burned gas, also harmful to health)
- CH: Hydrocarbons (unburned gasoline particles)
- O2: Oxygen particles (that did not participate in combustion)
- NOx: Nitrogen compound (formed only at very high combustion temperatures).
The catalyst converts the 3 harmful components CO, HC, and NOx into 3 non-harmful components: CO2, H2O, and N2. This is where the name three-way catalyst comes from.
To add O2 and CO to the catalyst to allow the conversion to take place, the engine’s injection pattern must be adjusted. To form O2, the mixture must be lean (less fuel, more air). To form CO, the mixture must be rich (more fuel, less air). This is not the case with lean-burn engines, see the NOx catalyst section further down the page.
By injecting slightly too much and slightly too little fuel into the cylinders, a rich and lean mixture is alternately created. The excess CO and O2 thus end up in the catalyst. In the catalyst, platinum reacts with the CO and HC. Rhodium provides the reduction of the NOx. That also explains why varying voltage is measured when measured on the Lambda sensor. The voltage varies between 0.2 and 0.8 Volts (from lean to rich, etc.). The car’s engine management system (the ECU) regulates this itself. No adjustments need to be made.
| Harmful substance: | Addition of: | Results in: |
| CO + | O2 = | CO2 |
| HC + | O2 = | CO2 + H2O |
| NOx + | CO = | N2 + CO2 |
To add O2 and CO to the catalyst so that conversion can take place, the injection pattern of the engine must be adjusted. To form O2, the mixture must be lean (less fuel, more air). To form CO, the mixture must be rich (more fuel, less air). This is not the case with lean-burn engines, see the NOx catalyst chapter further down the page.
By injecting slightly too much and too little fuel into the cylinders, a rich and lean mixture alternates. The excess CO and O2 thus end up in the catalyst. In the catalyst, platinum reacts with the CO and HC. Rhodium takes care of the reduction of the NOx. That explains why a variable voltage is measured when measuring on the lambda sensor. The voltage varies between 0.2 and 0.8 Volts (from lean to rich, etc.). The car’s engine management system (the ECU) regulates this itself. No adjustments are required.
What can be seen in the table above is that the substances are all converted to, among others, CO2. CO2 is now considered a substance that is dangerous for the environment and responsible for global warming. However, a person also exhales CO2. This is converted back into O2 (oxygen) by trees and plants. An excess of CO2 has a harmful impact. Trees and plants are in the minority and cannot convert everything back into O2. For internal combustion engines, the CO2 content should be as high as possible. That sounds odd because one would think it should be kept as low as possible. Here’s the thing; the higher the CO2 content, the less CO and HC are released. CO and HC are directly harmful to health when inhaled. The only way to reduce the CO2 content is by switching to alternative fuels, smaller (more efficient) combustion engines, and more moderate driving habits.
Operating Temperatures:
The beneficial operation of the catalyst starts at a temperature of 250 degrees and is maximum at a temperature of 450 degrees. After starting the engine, it takes a while before the purifying action begins. The catalyst is mounted as close as possible to the exhaust manifold so that it reaches its working temperature sooner. Exhaust gas temperatures between 800 and 1000 degrees cause faster thermal aging, shortening the lifespan and reducing the active surface area.
There are also catalysts with a heating element that ensures that the catalyst reaches temperature even faster after a cold start. It can then regulate even faster after the engine is started, resulting in cleaner exhaust gases.
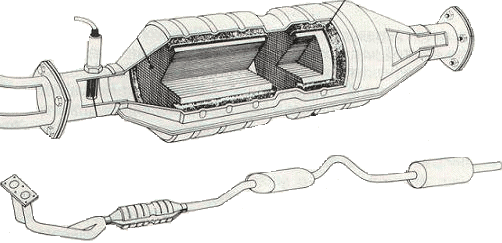
To warm up the catalyst as quickly as possible after a cold start, a secondary air pump is often used.
Operation of NOx Catalyst:
It was previously explained that NOx can be reduced by the catalyst by obtaining extra CO in the exhaust gas. This is only possible by making the mixture richer. With lean-burn engines from, for example, Volkswagen (FSI) and BMW (Efficient Dynamics), the engines always run on a mixture with an excess of air (so lean, and never rich) during part load and low revs. With a normal three-way catalyst, it is therefore impossible to convert NOx to N2 + CO2. To still remove the NOx from the exhaust gases, a special NOx (storage) catalyst with a special barium component is required. In addition to the barium component, this catalyst also contains noble metals such as platinum and rhodium.
The three-way catalyst converts the CO and HC values into CO2 and H2O as previously described. The NOx is converted by the NOx catalyst. Extra temperature sensors and a NOx sensor are needed to constantly check the values.
In the image below, an exhaust system is seen as applied in, for example, VW, BMW (and increasingly more brands).
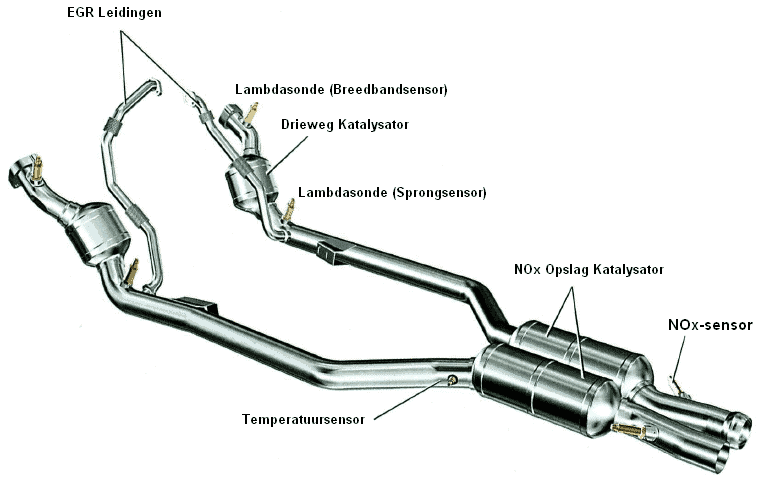
The NOx gases are stored in cold conditions in this catalyst. The other exhaust gases can continue their way through the exhaust. During the oxygen-rich period, the NOx gases are stored in the barium component. The NOx accumulates (just as soot is stored in a particulate filter). Eventually, the catalyst becomes saturated. That is when it is full of NOx. The catalyst must then be regenerated. The NOx sensor recognizes this and sends a signal to the ECU. At this point, the mixture is enriched, specifically to regenerate the NOx catalyst. This only happens when the NOx catalyst has reached a temperature of 800 degrees (this is registered by the temperature sensor and also communicated to the engine control unit). With the temporary enrichment, extra CO is released. With the help of this CO, the platinum and rhodium components can convert to N2 + CO2. After regeneration, the engine will run on a lean mixture again until the catalyst is saturated.
Malfunctions can also occur in this system. When only short trips are taken with the car (which is bad for the entire car anyway), the NOx catalyst will also not be able to reach its working temperature. As soon as it is saturated (full), it must be regenerated. However, if the temperature sensor continues to measure too low a temperature, the ECU will never enrich the mixture. If the catalyst is not at operating temperature, the platinum and rhodium components cannot make a conversion. At this point, the engine malfunction light will illuminate, and the cause will come to the forefront when the car is read out. The catalyst should then be regenerated using the test box or a vigorous test drive. It is therefore best to drive a long stretch (e.g., 50 km or more on the highway) and preferably a stretch with an increased rpm. The catalyst will then easily reach its working temperature.
Nowadays, the SCR (Selective Catalytic Reduction) catalyst is used in diesel engines. This SCR catalyst also stores NOx, but an AdBlue dosing system is also added.
Aging and Causes:
- Gasoline: A three-way catalyst can only work with unleaded gasoline. If leaded gasoline is nevertheless used, it adheres in a thin layer to the noble metal, reducing contact with the exhaust gases and eventually making it even impossible. No chemical reaction can then occur. The catalyst is therefore out of operation and needs to be replaced. This is a costly affair. Leaded gasoline was added to achieve a certain knock limit. Nowadays, knock sensors are used, so the lead has been removed from the fuel.
- Oil also has a destructive effect on the interior. With a lot of oil leakage along, for example, the piston rings, valve guides, or the turbo, a lot of oil can end up in the catalyst. Also, the oil adheres in a layer to the noble metal, which then loses its effectiveness.
- Short trips: Driving many short trips rarely, if ever, brings the catalyst to its operating temperature. The unburned HC (gasoline) residues adhere to the ceramic surface. If long trips are taken, these HC residues will still burn. If only short trips are taken, these HC residues will adhere to the interior, causing the catalyst to eventually lose its effectiveness.
The second lambda sensor (the jump sensor) often measures whether the catalyst has effectively converted the gases. Should the catalyst age or the interior be defective, this second lambda sensor will measure it. A warning light will illuminate on the dashboard. Replacing the catalyst is then necessary. More information about the lambda sensor can be found on the page Lambda Sensor.
