Introduction:
The cooling process in a car’s air conditioning uses state changes of a substance. During a state change, such as the transition from liquid to vapor, the molecular structure of the substance changes, which requires heat. Heat is absorbed when liquid changes into vapor, and in the opposite case, when transitioning from vapor to liquid, heat is released.
When we look at heat transfer to and from the environment, we see that during the evaporation process the environment cools down, while heat is released and the environment warms during condensation. This cooling of the environment occurs in the evaporator, while heating takes place in the condenser. This process repeats continuously, which is why it’s known as a cycle process.
The “Air Conditioning Introduction” page describes the cycle process with the various components of the air conditioning in a practical manner. On this page, we will further explore this cycle process through the log pH diagram.
Cycle Process:
Before we show a complete log pH diagram, we first start with the cycle process of the air conditioning. In this cycle process, we use the diagram of the refrigerant R134a. In this diagram, the areas for gas, gas-liquid, and liquid are distinguished from one another. The critical point is at the top, at 101 degrees Celsius and a pressure of 40 bar. These are the maximum temperature and pressure at which the refrigerant is chemically stable. On the x-axis, the enthalpy (heat content) is plotted against the pressure. Although we often refer to a “pH diagram,” it is actually a “log pH diagram” due to the logarithmic scale.
- At point 1 in the diagram, the compressor starts, which draws in refrigerant from the evaporator. The pressure is 2 bar;
- The gas is compressed from 1 to 2, leading to an increase in pressure and heat content. The pressure and temperature rise to 15 bar and 70 degrees Celsius. The gas becomes superheated;
- Due to heat release in the condenser, the heat content initially decreases, and thus the temperature. The gas loses its superheat between point 2 and 3, causing the temperature to drop from 70 to 55 °C;
- From point 3 to 4, heat is discharged at a constant temperature. Here, the gas is converted into liquid. The pressure remains constant;
- With further cooling, the liquid becomes slightly subcooled (from 4 to 5). The subcooled liquid under the high pressure of 15 bar reaches a constriction at point 5: the capillary or expansion valve. Here, the high pressure is separated from the low pressure. From the compressor’s perspective, we can also say that the discharge pressure is separated from the suction pressure.
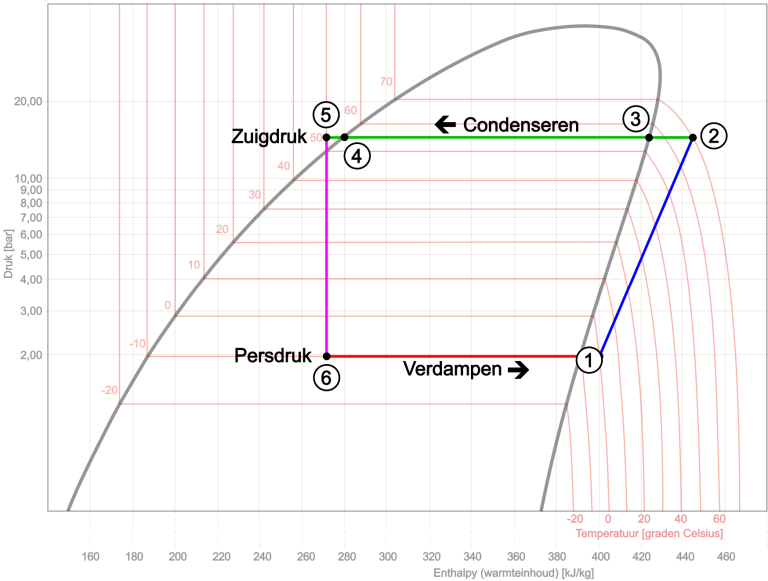
Due to the sudden pressure drop in the narrowing, the boiling point of the refrigerant in its liquid phase will be lower, causing spontaneous evaporation. The heat required for this is first taken from the refrigerant itself and its nearby environment. The heat content remains almost constant. Subsequently, complete evaporation takes place from point 6 to 1 in the evaporator. 0The boiling temperature of the refrigerant drops between point 5 and 6 from 50a0°Ca0to -10 °C, eventually warming up as a gas to 0 °C at point 1.a0The heat content of the refrigerant increases, with the required heat extracted from the surroundings, in this case, the passing air through the evaporator. Pressure and temperature remain almost constant. As vapor, the refrigerant exits the evaporator and is drawn in again by the compressor at point 1. The process repeats.
Log pH Diagram:
In the previous section, the log pH diagram was shown, depicting the cycle process (from evaporating to condensing the refrigerant. The image below shows the condition of the refrigerant at a certain pressure in relation to the enthalpy (heat content), with the cycle process indicated by the dark blue line.a0
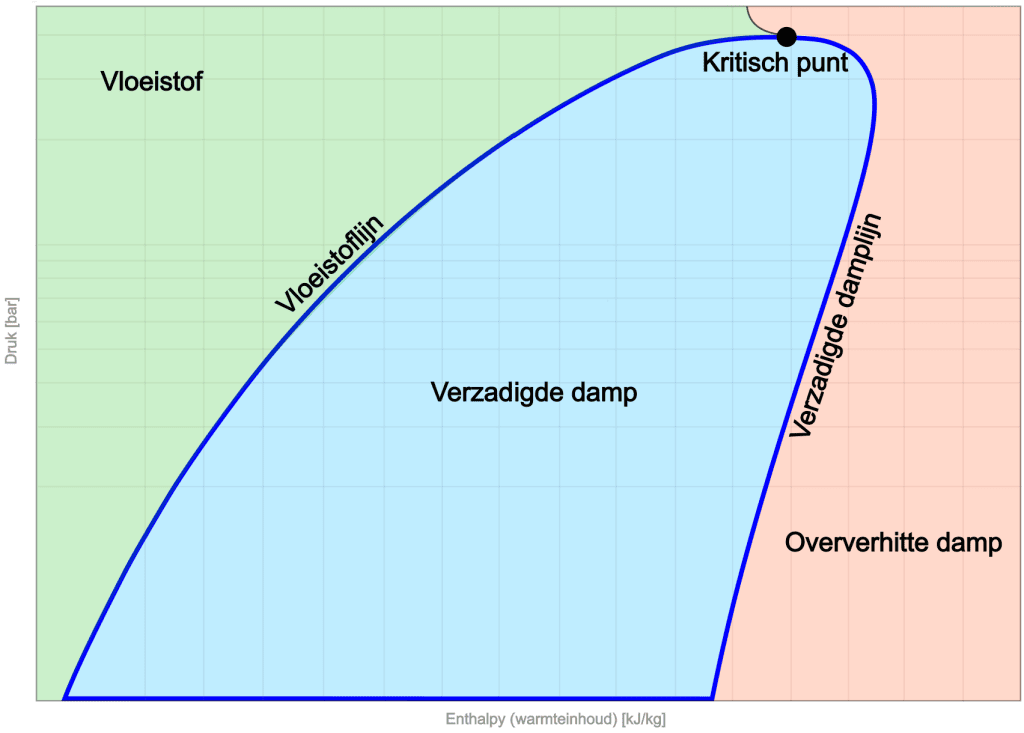
On the left side of the diagram is the liquid area. At low enthalpy, the refrigerant is in liquid form. With increasing enthalpy, the liquid line is reached. The slope of this line indicates changes in pressure and enthalpy for the liquid phase.
In the middle of the diagram is the saturated vapor zone. Here, the refrigerant is in thermal equilibrium, with both liquid and vapor present.
On the right side, we see the saturated vapor line, which marks the boundary where the refrigerant has fully evaporated and is in a superheated vapor phase.
At the top of the diagram is the critical point, marking the boundary between liquid and vapor. Here, the distinction between vapor and liquid phases disappears, placing the refrigerant in a unique state. There is no clear transition between liquid and vapor.
To provide more insight into the log pH diagram, multiple curves are added to the diagram below: the isentropic, isothermal, isochoric, and vapor quality. In the drawing below, we again see the cycle process (gray colored) with the course of the other processes. Here is a brief explanation per state change:
Isentropic:a0The isentropic line is characterized by constant entropy. This means that the refrigerant undergoes no heat exchange with the environment and no change in entropy during a process along this line. It is an efficient adiabatic (without heat exchange) process line in the diagram.
Isothermal: An isothermal line in the log pH diagram represents a constant temperature process. During this process, the temperature of the refrigerant remains constant, meaning heat is added or removed to keep the pressure-enthalpy (pH) ratio constant.
Isochoric: An isochoric line in the log pH diagram represents a constant volume process. During this process, the specific volume of the refrigerant remains constant, meaning no change in volume occurs. As a result, the line can slope steeply up or down in the diagram, depending on other changes such as pressure and enthalpy.
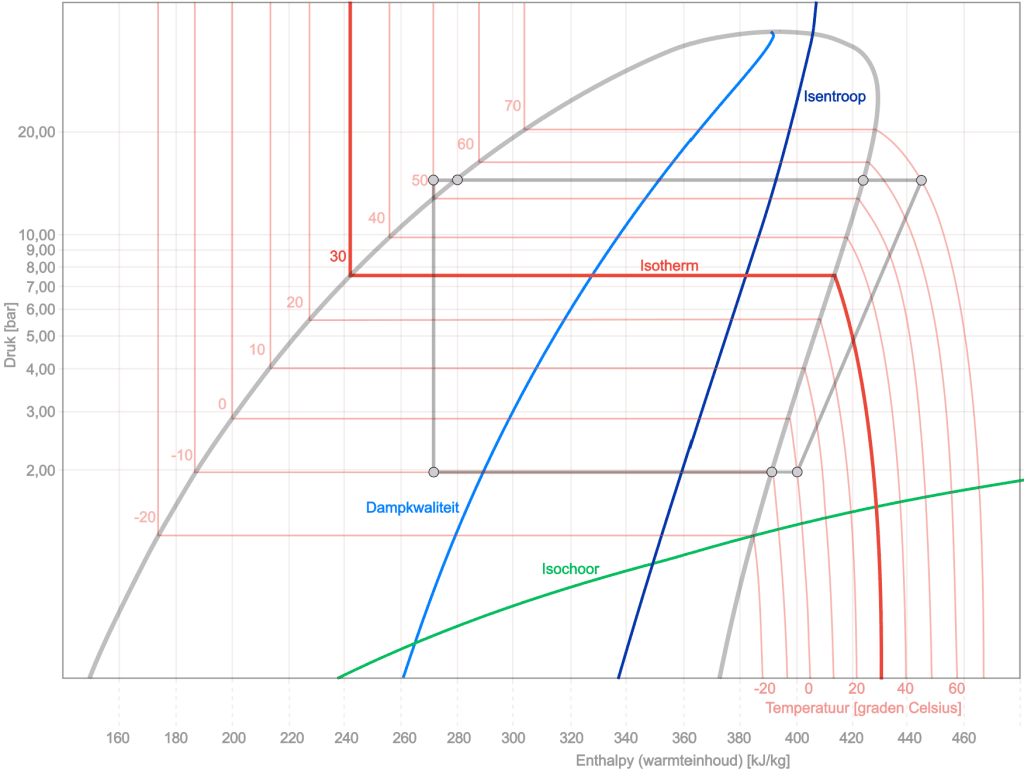
- Vapor Quality: In a log pH diagram of refrigerant, the x-axis indicates the quality range, from “x=0” (fully liquid) to “x=1” (fully gaseous). Between these extremes, the refrigerant is in a two-phase state, where the x-value indicates the ratio of gas to liquid. A line from “x=0.10” to “x=0.90” in the diagram shows that the refrigerant is within this two-phase range, with the specific x-value indicating the distribution between gas and liquid. This is crucial for understanding the behavior of the refrigerant in applications like refrigeration and air conditioning systems.
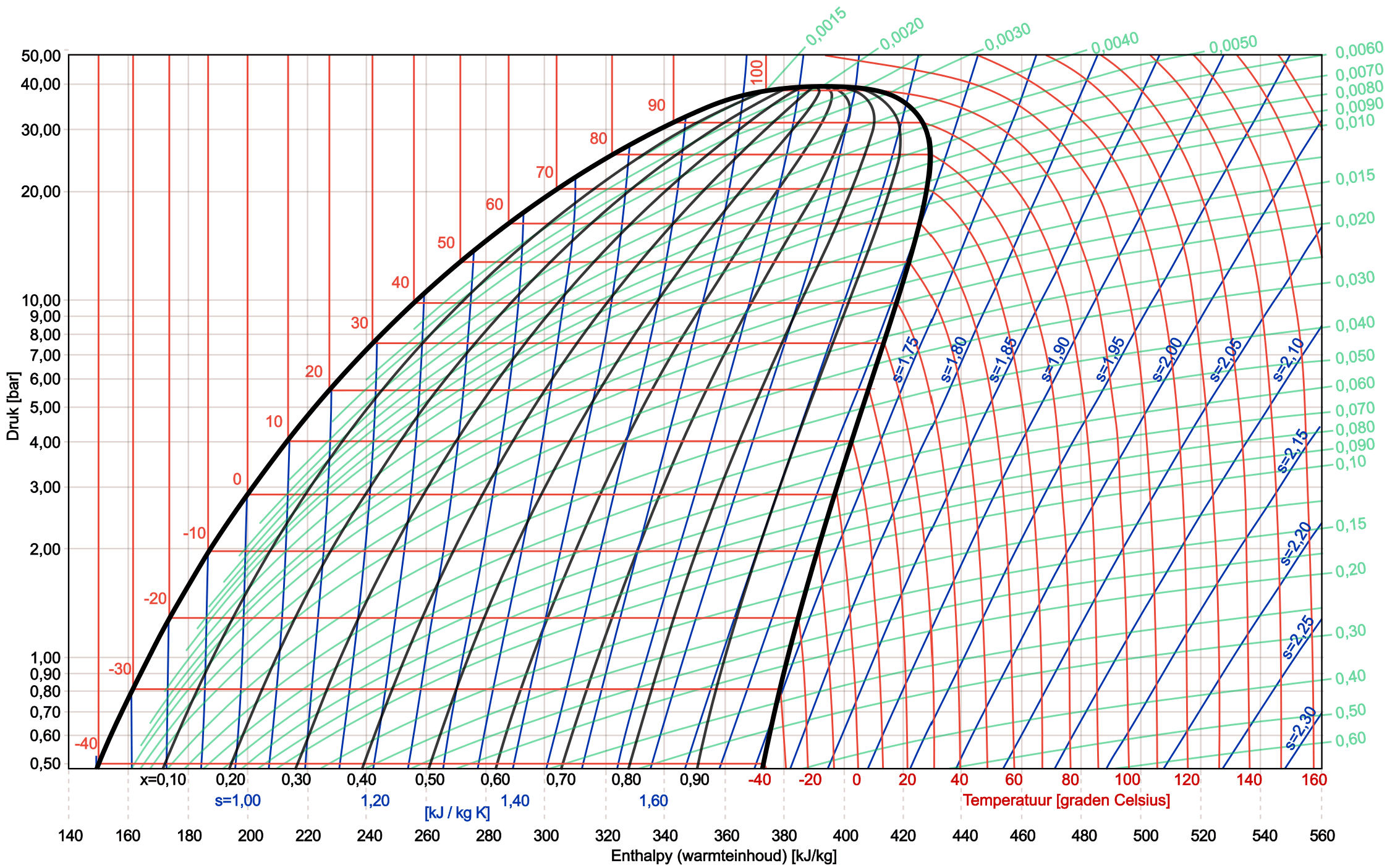
In the image below, we see a complete log pH diagram of the refrigerant R134a.
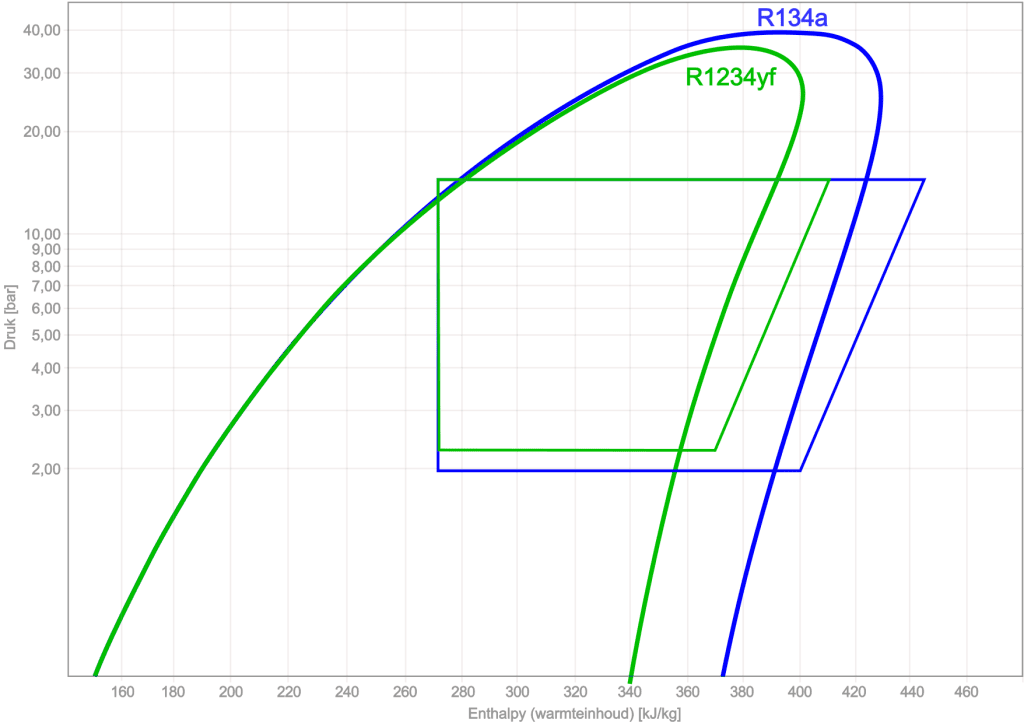
Comparing R134a with R1234yf:
Using the log pH diagram, different types of refrigerants can be compared. The following image shows the log pH diagrams and cycle processes of R134a and R1234yf.
Related page:
