Subjects:
- Hydrogen
- Production of hydrogen
- Hydrogen as fuel for an Otto engine
- Fuel cell
- Storage tank
- Range and costs of hydrogen
Hydrogen:
Hydrogen (called hydrogen in English) can be used as an energy carrier to power vehicles. Energy carrier means that an energy in the hydrogen has already been put into it beforehand. This is in contrast to (fossil) energy sources such as petroleum, natural gas and coal, where the energy is obtained by processing these substances by burning them.
Hydrogen is therefore something completely different from water injection, which is not used as an energy carrier in petrol engines, but purely as a cooling system for the combustion chamber.
The aim is to achieve “zero emissions” with hydrogen; a form of energy in which no harmful gases are formed during use. The transition from fossil fuels to electric propulsion in combination with hydrogen and a fuel cell falls under the energy transition. Hydrogen vehicles can be powered in two different ways:
- Using hydrogen as fuel for the Otto engine. The hydrogen replaces the gasoline fuel.
- Generating electrical energy using hydrogen in a fuel cell. Using this electrical energy, the electric motor will drive the vehicle fully electrically.
Both techniques are described on this page.
Hydrogen can be produced with sustainable energy or on the basis of fossil fuels. We try to prevent the latter as much as possible, because fossil fuels will become scarce in the future. CO2 will also be produced during the processing of fossil fuels.

The columns below show the energy content of a battery, hydrogen and gasoline. In it we see that there are many
Battery:
- Energy content: 220Wh/kg, 360 Wh/l
- Very efficient
- Short storage
- Immediate energy delivery possible
- Transportation is complicated
Hydrogen (700 bar):
- Energy content: 125.000 kJ/kg, 34,72 kWh/kg
- 30% heat, 70% H2 (PEM fuel cell)
- Long storage possible
- Conversion necessary
- Transport friendly
Petrol:
- Energy value: 43.000 kJ/kg, 11,94 kWh/kh
- Return up to 33%
- Long storage possible
- Conversion necessary (combustion)
- Transport friendly
Hydrogen is all around us, but never released. It is always bound. We will produce, isolate and store it.
- 1 kg pure hydrogen (H2) gas = 11.200 liters at atmospheric pressure
- H2 is smaller than any other molecule
- H2 is lighter than any other molecule
- H2 is always looking for connections
In addition to the production and application of hydrogen in passenger cars, this page also discusses storage and transport (at the bottom of the page).
Production of hydrogen:
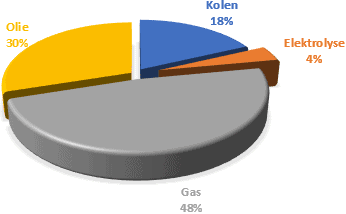
Hydrogen is a gas that is not extracted from the ground, like natural gas. Hydrogen has to be produced. This is done through electrolysis, a process in which water is converted into hydrogen and oxygen. That is the reverse of the reaction that takes place in a fuel cell. In addition, hydrogen can be obtained via less environmentally friendly processes. In the data below we see how hydrogen can be produced in 2021.
- Coal: C + H20 -> CO2 + H2 + Nox + SO2 + … (temp: 1300C-1500C)
- Natural gas: CH4 + H2O -> CO2 + 3H2 (required temp: 700C-1100C)
- Oil: CxHyNzOaSb + …. -> cH2 + a lot of by-products
- Electrolysis from water: 2H2O -> 2H2 + O2
Electrolysis from water is very clean and is the most environmentally friendly form of hydrogen production. This releases hydrogen and oxygen, in contrast to the processing of fossil fuels, which releases CO2.
- electrolysis of water; Electrolysis is a chemical reaction in which water molecules are split to create pure hydrogen and oxygen. Hydrogen can be produced wherever there is water and electricity. A disadvantage is that you need electricity to make hydrogen and then turn it into electricity again. Up to 50% is lost in this process. The advantage is that the energy is stored in hydrogen.
- Converting fossil fuels; oil and gas contain hydrocarbon molecules made up of carbon and hydrogen. Hydrogen can be split from the carbon with a so-called fuel processor. The downside is that the carbon disappears into the air as carbon dioxide.
We call the hydrogen production obtained with fossil fuels gray hydrogen. Among other things, NOx and CO2 end up in the atmosphere.
From 2020, production will become increasingly “blue”: CO2 is captured.
The aim is to produce exclusively green hydrogen by 2030: green electricity and water are the sources for the most environmentally friendly generated hydrogen.
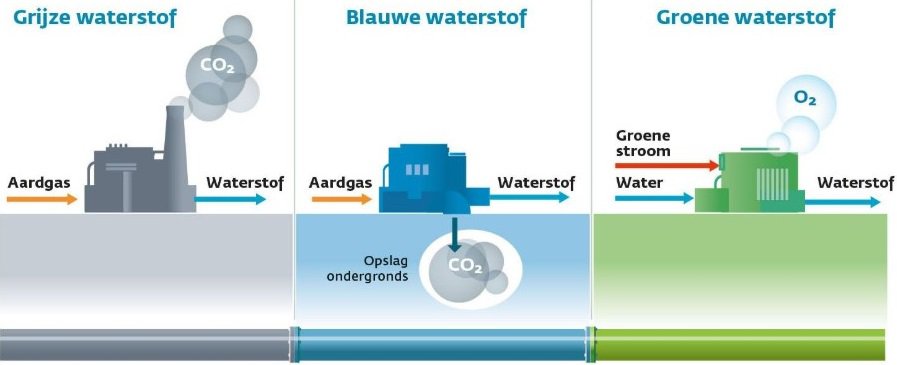
In the chemical world, hydrogen is indicated by H2, which means that a hydrogen molecule is made up of two hydrogen atoms. H2 is a gas that does not occur in nature. The H2 molecule is found in all kinds of substances, the most famous of which is water (H20). Hydrogen must be obtained by loosening the hydrogen molecule from, for example, a water molecule.
Producing hydrogen by means of electrolysis is therefore the future.
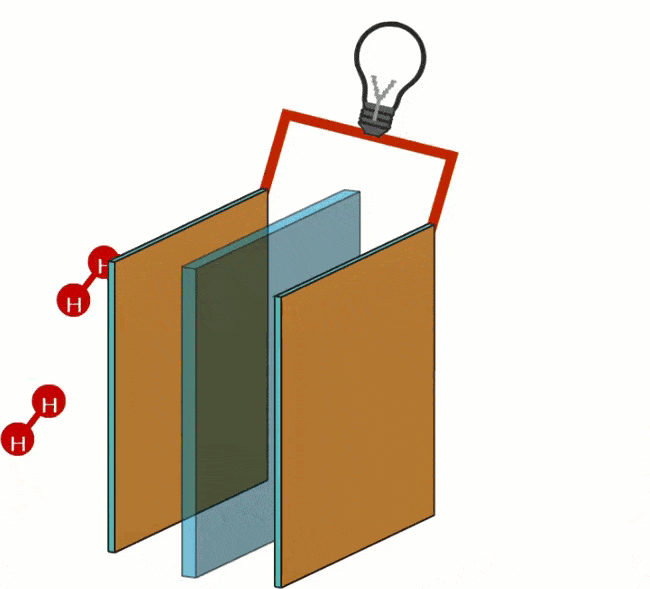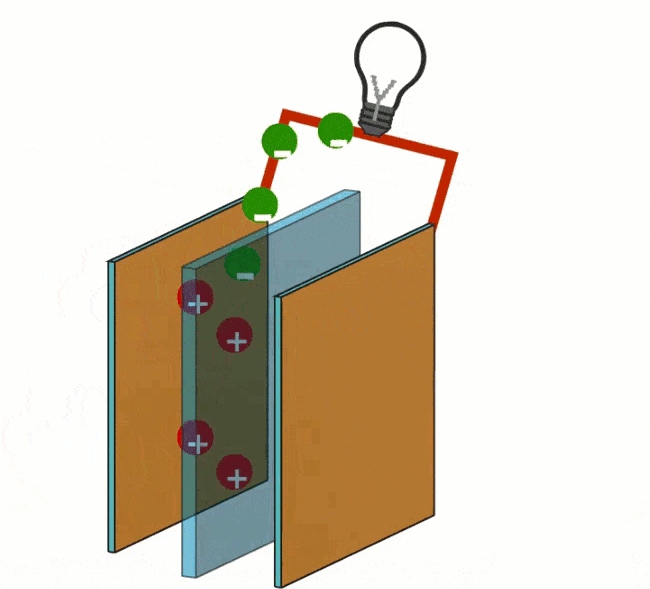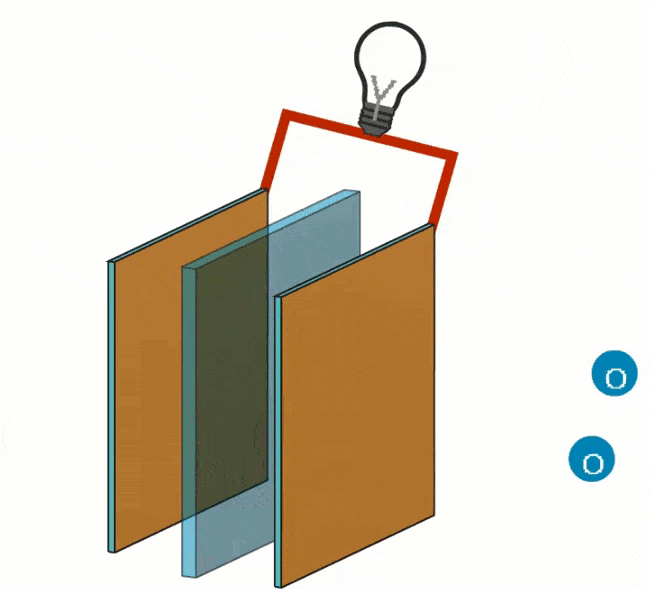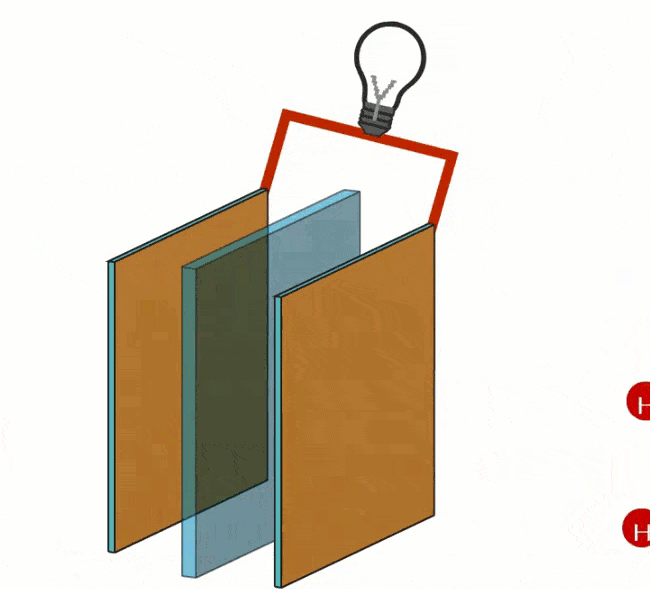
The following image shows a model often used in chemistry classes.
- Plus and minus bars of a battery hang in the water;
- On the anode side you get oxygen;
- On the cathode side you get hydrogen.
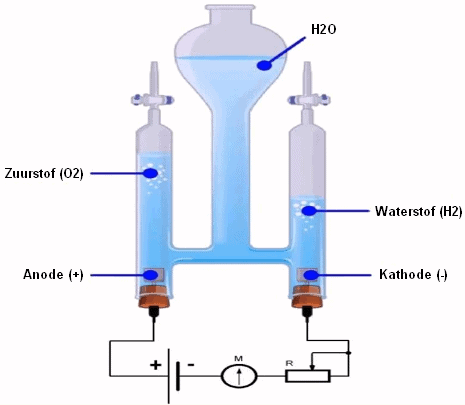
Hydrogen produced from fossil fuel, for example Methane (CH4) is in this case converted into H2 and CO2 via reforming. The CO2 can be separated and stored underground in, for example, an empty natural gas field. Thus, the use of natural gas makes little or no contribution to the emission of CO2 into the atmosphere. Hydrogen can also be made from biomass. If the CO2 released in this process is also separated and stored underground, it is even possible to achieve negative CO2 emissions; extracting CO2 from the atmosphere and storing this CO2 on Earth.
In contrast to fossil fuels such as petroleum, natural gas and coal, hydrogen is not an energy source, but an energy carrier. This means that the energy that is released when hydrogen is used, for example as fuel in a car, must first be put into it. Electricity is required for the production of hydrogen by means of electrolysis. The sustainability of this hydrogen then largely depends on the sustainability of the electricity used.
Hydrogen as fuel for an Otto engine:
An Otto engine is another name for a petrol engine. The petrol engine was invented in 1876 by Nikolaus Otto. In this case we call it an Otto engine, because the petrol is replaced by another fuel, namely hydrogen. In an engine where hydrogen is injected, there is no longer a fuel tank with petrol.
When hydrogen is burned, no CO2 gases are created, unlike conventional Otto and diesel engines, but only water. When hydrogen is injected by direct injection, there will be a power increase of 15 to 17% compared to the gasoline fuel. When the hydrogen is injected onto the inlet valve (in case of indirect injection), the air quickly heats up. The air is also displaced by the hydrogen. In both cases, less oxygen (O2) flows into the combustion chamber. In the worst case, there is a power loss of up to 50%.
The ratio between air and hydrogen is not as strict as, for example, an air-petrol mixture. The shape of the combustion chamber is therefore not of great importance.
Hydrogen can be injected in two ways:
– Liquid: With liquid supply of hydrogen, the combustion temperature will drop relatively due to evaporation, so that less NOx is created.
– Gaseous: When the hydrogen is stored in liquid form in the tank and it enters the combustion chamber at ambient temperature, an evaporator must be used to convert the hydrogen from liquid to gaseous state. In this case, the evaporator is heated by the coolant from the engine. Possible measures to reduce the NOx are; applying EGR, water injection or a lower compression ratio.
The figure below shows four situations with three different versions of hydrogen injection. In the second image from the left, the gaseous hydrogen is indirectly injected into the intake manifold. The gaseous hydrogen is heated by the ambient temperature. The hydrogen also takes up space, which means that less oxygen can flow into the cylinder. This is the situation where the most power loss is obtained.
In the third image, the hydrogen is supplied in liquid form. Cryogenic means that the hydrogen has been cooled very strongly (a method of storing large amounts of hydrogen in liquid form in a relatively small storage tank). Because the temperature of the hydrogen is lower and it is in a liquid state, a better cylinder filling takes place. Due to the low temperature, an efficiency that is almost as high as an engine with direct (hydrogen) injection is achieved. The direct injection engine can be seen in the fourth image. The entire combustion chamber is filled with oxygen. When the intake valve is closed and the piston is compressing the air, a certain amount of hydrogen is injected through the injector. The spark plug is located behind or next to the injector on this engine (it is not shown in the picture).
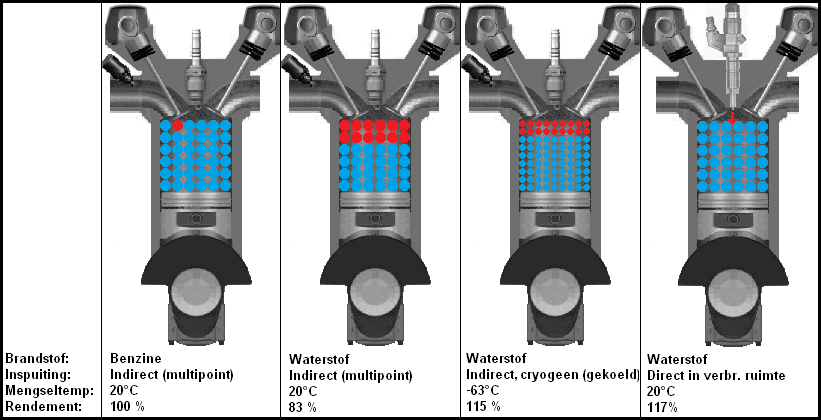
The efficiency of an Otto engine is of course not 100%, but in this image the efficiencies of the combustion of hydrogen are compared with the combustion of gasoline.
Hydrogen has a high energy density per unit mass (120MJ/kg), making it almost three times higher than gasoline. The good ignition readiness of hydrogen makes it possible to run the engine very lean, with a lambda value of 4 to 5. The disadvantage of using a lean mixture is that the power will be lower and the driving characteristics will be less. To compensate for this, supercharging is often used (a turbo).
Due to the larger ignition area compared to gasoline fuel, the risk of detonation or backfire is greater. It is therefore very important that there is good regulation of the fuel supply and ignition. At full load, the temperature in the combustion chamber can rise very high. There is often water injection necessary to ensure sufficient cooling, and thus also to prevent premature ignition (in the form of detonation or a backfire).
Fuel cell:
The previous section explained how hydrogen can serve as fuel for the combustion engine. Another application of hydrogen is in the fuel cell. A vehicle equipped with a fuel cell does not have a combustion engine but one or more electric motors. The electrical energy to run the electric motors is produced by the fuel cell. A fuel cell is an electrochemical device that converts chemical energy directly into electrical energy, without thermal or mechanical losses. The energy conversion in the fuel cell is therefore very efficient. In general, the fuel cell works on hydrogen, but a fuel such as methanol can also be used for this.
In principle, a fuel cell can be compared to a battery, because both produce electricity through a chemical process. The difference is that the energy stored in the battery is released once. The energy runs out over time, so the battery has to be recharged. A fuel cell supplies continuous energy as long as reactants are supplied to the electrochemical cell. Reactants are chemicals that react to each other in a chemical reaction.
In a fuel cell, hydrogen and oxygen are converted into H+ and OH- ions (particles that are charged). The ions are separated in separate chambers of the fuel cell by a membrane. The fuel cell contains two porous carbon electrodes on which a catalyst is applied; with hydrogen (H) a negative electrode (anode) and with oxygen (O) a positive electrode (cathode).
H+ and OH- ions are led to each other via the electrodes (anode and cathode), after which the + and – ions react with each other. The cathode catalyzes the reaction where the electrons and protons react with oxygen to end product two, namely water. The H+ and OH- ions together form an H2O molecule. This molecule is not an ion, because its electrical charge is neutral. The plus particle and the minus particle together gives a neutral particle.
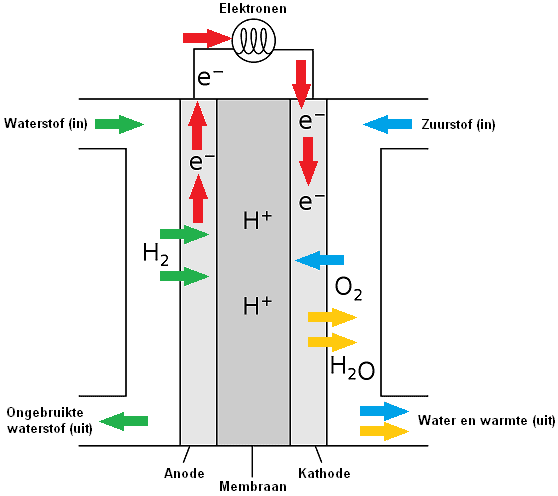
At the anode, the oxidation of the hydrogen (H) takes place. Oxidation is the process by which a molecule loses its electrons. The anode acts as a catalyst, splitting the hydrogen into protons and electrons.
Reduction takes place at the cathode by adding oxygen (O). The electrons, closed off by the anode, will travel to the cathode via an electrical wire that connects the electrons on the outside.
By not allowing the electron transfer to take place directly, but via an external route (the current wire), this energy is largely released as electrical energy. The circuit is closed by ions in a connecting electrolyte between reducing agent and oxidizing agent.
The particle that takes up electrons is called an oxidizer and is thereby reduced. The reductor loses electrons and is oxidized. A reduction is the process by which a particle gains electrons. Oxidation and reduction always go together. The number of electrons donated and taken up is always the same.
The following reaction takes place at the negative pole:
A different reaction takes place at the positive pole:
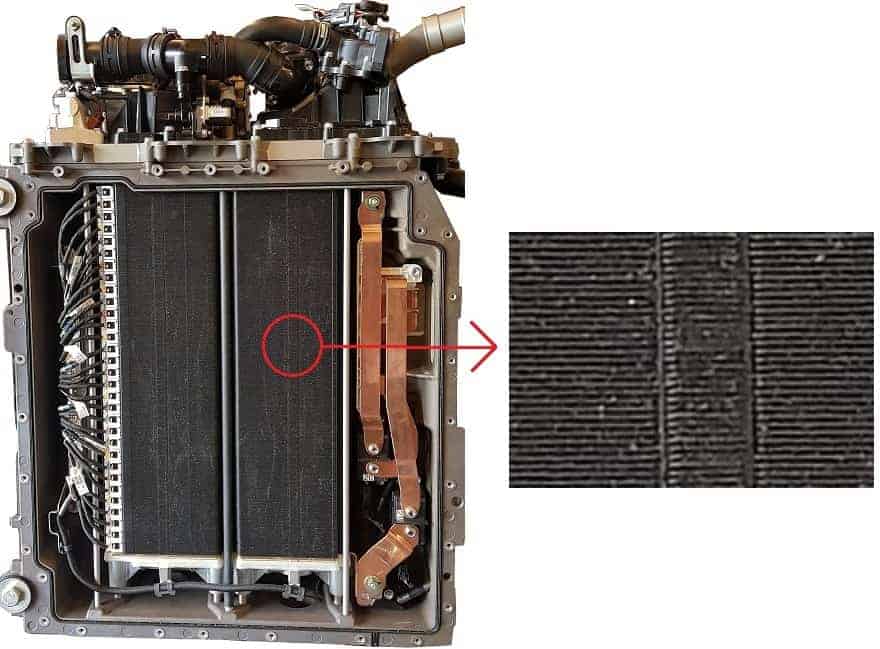
The image below shows the bottom view of a Toyota fuel cell stack. This fuel cell stack is located under the hood of the car. The electric motor is attached to this stack. The electric motor supplies the power to the transmission, which is connected to the drive shafts to transfer the driving forces to the wheels.
Various air tubes can be seen at the top of the stack. This includes the air pump that pumps the air to the fuel cells, depending on the power required by the electric motor.
This fuel cell stack is equipped with 370 fuel cells. Each fuel cell supplies 1 volt, so a total of 370 volts can be supplied to the electric motor. The fuel cells are all located underneath each other. The red circle shows an enlargement, where the accumulation of the fuel cells is clearly visible.
Storage tank:
Although hydrogen has a high energy density per unit mass (120MJ/kg), almost three times as high as petrol, the energy density per unit volume is very low due to the lower specific mass. For storage, this means that the hydrogen must be stored under pressure or in liquid form in order to be able to use a storage tank that is manageable in terms of volume. There are two variants for vehicle applications:
- Gaseous storage at 350 or 700 bar; at 350 bar, the tank volume in terms of energy content is a factor of 10 greater than with petrol.
- Liquid storage at a temperature of -253 degrees (cryogenic storage), in which case the tank volume in terms of energy content is a factor of 4 larger than with petrol. With gaseous storage, hydrogen can be stored indefinitely without loss of fuel or quality. Cryogenic storage, on the other hand, creates vapor formation. Because the pressure in the tank increases due to heating, hydrogen will escape through the pressure relief valve; a leakage of about two percent per day is acceptable. Alternative storage options are still in a research stage.
The image below shows two storage tanks under the car. These are storage tanks in which the hydrogen is stored in gaseous form under a pressure of 700 bar. These storage tanks have a wall thickness of approximately 40 millimeters (4 centimeters), making them resistant to the high pressure.

Below you can see once again how the hydrogen tanks are mounted under the car. The plastic tube is the drain of water that is created during the conversion in the fuel cell.

Refueling with hydrogen:
There are only two hydrogen filling stations in the Netherlands at the time of writing this article. One of these gas stations is located in Rhoon (South Holland). The images show the filling pistols used for refueling. The filling operating pressure is 350 bar for commercial vehicles and 700 bar for passenger cars.


The filling connection in the car is located behind the usual fuel filler flap. The filling pistol is connected to this filling connection. After connecting the filling pistol, the connection will lock. The car's storage tank will be filled with gaseous hydrogen at a pressure of 700 bar.


Range and costs of hydrogen
As an example, let's take a Toyota Mirai (model year 2021) and look at the range and the additional costs:
- Range of 650 km;
- Consumption: 0,84 kg / 100 km;
- Fuel price per km: 0,09 to 13 cents;
- Road tax €0,-
Compared to a vehicle with a diesel engine, a fuel cell car is not cheap. Although the costs of road tax play a major role, the number of filling stations in the Netherlands in 2021 is still scarce. Below is a comparison of the costs per 100 km with the current fuel prices:
BMW 320d (2012)
- Diesel: €1,30 per litre;
- Consumption: 5,8 l/100 km;
- Costs 100 km: €7,54.
Toyota Mirai (2020):
- Hydrogen: €10 per kg;
- Consumption: 0,84 kg/100 km;
- Costs 100 km: €8,40
Related pages:
- Electric drive (Overview);
- Energy transition.
