Subjects:
- Preface
- Materials and specifications of different batteries
- lead-acid battery
- Nickel Cadmium (Ni-Cd)
- Nickel Metal Hydride (Ni-MH)
- Lithium ion (li-ion)
- Super capacitor (super cap)
- Battery cell balancing
Preface:
The hybrid or fully electric car has larger, heavier batteries than cars with only a combustion engine. Hybrid cars work with high voltages, which can be life-threatening when repaired by inexperienced people. As an example:
- A starter motor in operation uses around 1,2 kW (1200 Watts)
- A hybrid car that runs entirely on electricity uses around 60 kW (60.000 Watts)
Hybrid cars may only be worked on by people who have undergone special training. There is a 12-volt on-board network for the power supply of accessories (such as radio, etc.) with its own small battery, and there is a high-voltage on-board network that (depending on brand) works at 400 volts. The 400 V voltage is converted to 12 V by a special DC/DC converter and charges the relevant battery.
High demands are placed on the hybrid drive batteries. They must be very large storage capacity to have. Large energy reserves are stored, and very high voltages are drawn when supporting the internal combustion engine (hybrid), or when delivering energy for the complete drive (BEV).
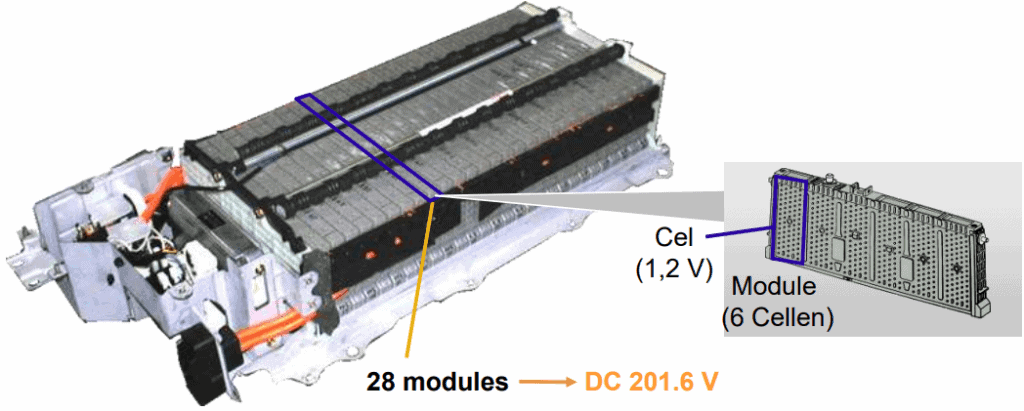
The image below shows a Toyota Prius battery pack. This Nickel Metal Hydride (NiMH) battery contains 28 modules, each consisting of 6 cells. Each cell has a voltage of 1,2 volts. In total, the voltage of this battery pack is 201,6 volts.
Materials and specifications of different types of batteries:
When developing the electric powertrain, a choice is made between different types of batteries. The properties, performance, construction options and costs play a major role in this. The most commonly used battery types in hybrid and fully electric vehicles are the Ni-MH (nickel-metal hydride) and the Li-ion (lithium-ion) batteries.
In addition to the Ni-MH and Li-ion types, electrolytic capacitors are being developed, which we place under the heading of “super-capacitor”, or “supercaps”.
The table shows the materials of the different batteries with their specifications.
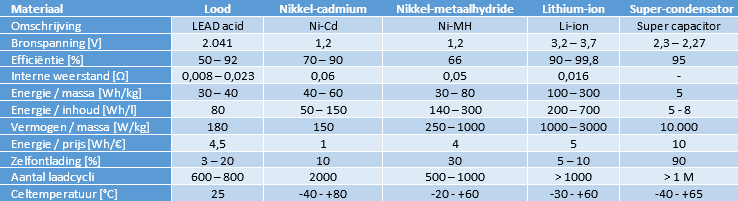
Lead battery:
The table also mentions the lead-acid battery (gel and AGM versions are not taken into account). Because the lead-acid battery has the longest lifespan with a maximum discharge of 20%, suffers from sulfation when aging and has a low energy density and content, it is not suitable for use in electric vehicles. We do find the lead battery as an accessory battery; the low-voltage consumers such as the lighting, comfort systems (body) and infotainment operate at a voltage of around 14 volts.

Nickel Cadmium (Ni-Cd):
In the past, Ni-Cd batteries suffer from a memory effect and are therefore already unsuitable for use in electric propulsion: partial charging and discharging takes place constantly. Modern Ni-Cd batteries are virtually no longer affected by the memory effect. The main disadvantage of this type of battery is the presence of the toxic substance cadmium. This makes the Ni-Cd battery extremely environmentally unfriendly. The use of this battery is therefore prohibited by law.

Nickel Metal Hydride (Ni-MH):
The Ni-MH battery can be charged faster than a lead battery. During charging, both heat and gas are generated, which must be removed. The batteries are equipped with a cooling system and vent valve. Thanks to its long service life and high energy and power density, the Ni-MH battery is suitable for use in electric vehicles. However, this type of battery is sensitive to overcharging, excessive discharges, high temperatures and rapid temperature changes.
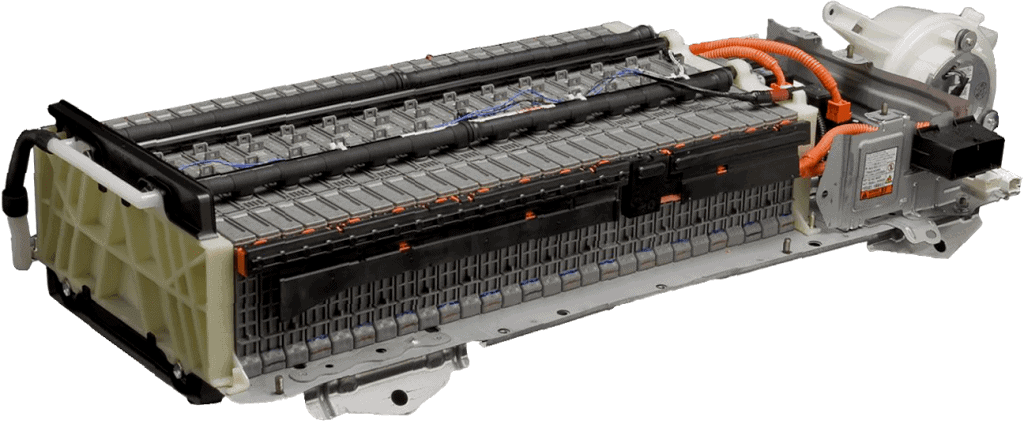
The image below shows the Ni-MH battery pack of a Toyota Prius. This battery pack is located in the trunk, behind the rear seat backrest. When the temperature sensors register a high temperature, the cooling fan is activated (shown in the photo on the right by the white housing). The fan sucks the air from the interior and blows it through the air ducts in the battery pack to cool the cells.
Lithium ion (li-ion):
Due to the high energy and power density of the lithium ion battery (compared to the Ni-MH), a li-ion battery pack is usually used in plug-in hybrids and fully electric vehicles. The Li-ion battery performs well at low temperatures and has a long service life. It is expected that the properties will improve in the coming years thanks to further development.
The following image shows the (li-ion) battery pack of a BMW i3. The lid is screwed off and is behind it. When mounted, the lid closes airtight.
The battery pack of the i3 is mounted under the vehicle. The space in the floor space between the front and rear axles has been used as much as possible to provide as much space as possible for the battery pack.
In the picture we see the eight separate blocks with twelve cells each. Each block has a capacity of 2,6 kWh, so that makes a total of 22 kWh. For comparison: the current generation i3 (in 2020) has a battery with a capacity of 94 Ah and a capacity of 22 kWh. The size of the battery pack has remained the same since its introduction in 2013, but the performance (and thus its range) has been greatly improved.

Tesla uses small battery cells in the models from 2013 (Model S and Model X) that are slightly larger than standard AA batteries that we know from the TV remote control. The battery cells (18650 from Panasonic) are 65 mm long and have a diameter of 18 mm. The most extensive battery packs contain as many as 7104 of these cells.
In the images below we see the separate battery cells on the left and a battery pack that houses the 7104 cells on the right.


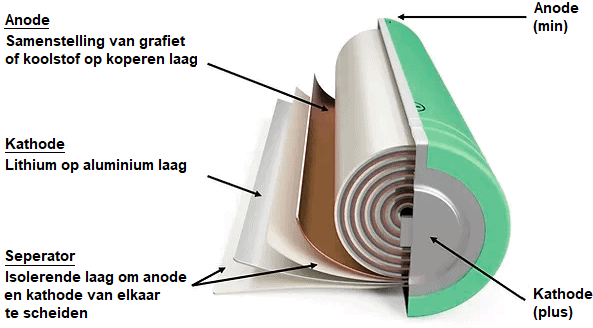
The lithium-ion battery is made up of four main components:
- the cathode (+) consisting of an alloy of lithium
- the anode (-) consisting of graphite or carbon
- the porous separator
- the electrolyte
During discharge, the lithium ions move through the electrolyte from the anode (-) to the cathode (+), to the consumer and back to the anode. When charging, the ions move in opposite directions and then move from the cathode (+) to the anode (-).
The electrolyte contains lithium salts to transport the ions. The separator allows the lithium ions to pass through, while the anode and cathode remain separated.
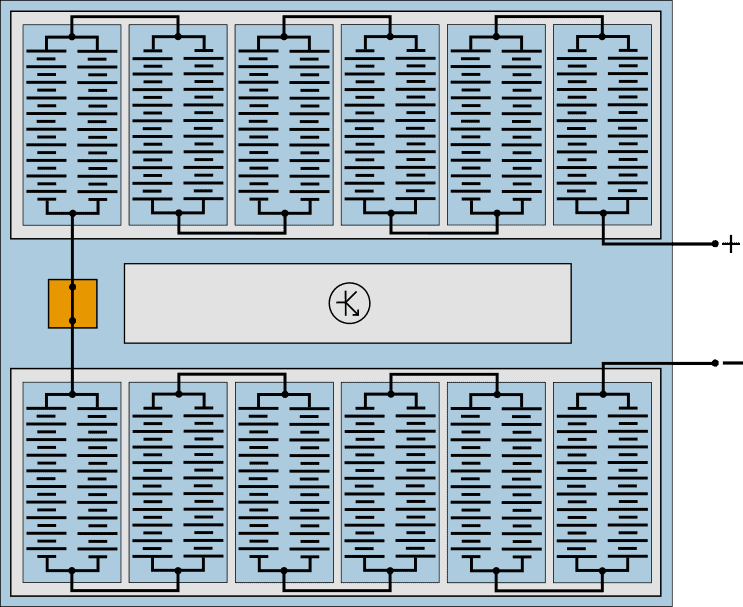
The battery cells are housed in modules, which are connected in series with each other. The following schematic diagram shows a battery pack that closely resembles that of a Volkswagen E-UP! and Renault Zoe. Only the number of cells differs: the battery pack of the E-UP! has 204 cells and that of the Renault Zoë 192.
In this example, the power pack consists of two packs of six modules. Each module contains two groups of 10 series switched cells connected in parallel.
- Series connection: the battery voltage increases. At a cell voltage (li-ion) of 3,2 volts, one battery module supplies (3,2 * 10) = 32 volts.
The disadvantage of a series connection is that with a bad cell, the capacity of the entire series connection decreases. - Parallel connection: the voltage remains the same, but the current and capacity increase. A bad cell will not affect the cells in the parallel circuit.
Manufacturers can therefore choose to use several parallel circuits per module. In the modules of the Volkswagen E-Golf, therefore, not two (as in this example), but three groups of cells are connected in parallel.
Lithium-ion cells have a lifespan of approximately 2000 discharge and charge cycles before their capacity is reduced to approximately 80% of their initial charge capacity.
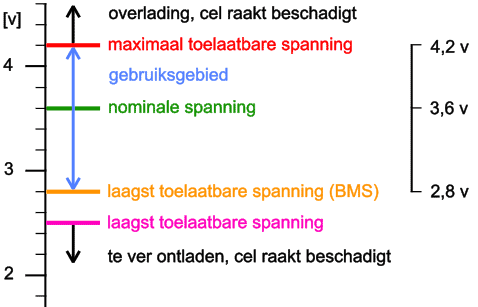
The voltages of a li-ion cell are as follows:
- nominal voltage: 3,6 volts;
- discharge limit: 2,5 volts;
- maximum charging voltage: 4,2 volts.
Most Battery Management Systems (BMS) use a lower limit of 2,8 volts. When the cell is discharged beyond 2,5 volts, the cell will be damaged. Cell life is shortened. Overcharging the li-ion cell also shortens its lifespan, but is also dangerous. Overcharging the cell can make it flammable. The temperature of the cells also influences the service life: at a temperature of less than 0°C, the cells may no longer be charged. A heating function offers a solution in this case.
Super encoder (supercap):
In the previous paragraphs, different battery types have been mentioned, each with their applications, advantages and disadvantages. A disadvantage that everyone with such a battery has to deal with is the charging time. Charging a battery pack can take several hours. Fast charging is an option, but that involves more heat and possibly also faster aging (and damage) of the battery pack.
A lot of research and development is currently taking place on super capacitors. We also call this the “super caps” or “ultracapacitors”. Applying supercaps could offer a solution for this:
- Charging is very fast;
- They can release (discharge) energy very quickly, so a significant increase in power is possible;
- More durable than a Li-ion battery thanks to an unlimited number of charge cycles (at least 1 million) because no electrochemical reactions occur;
- Partly in connection with the previous point, a supercap may be completely discharged without this having harmful consequences for the lifespan.
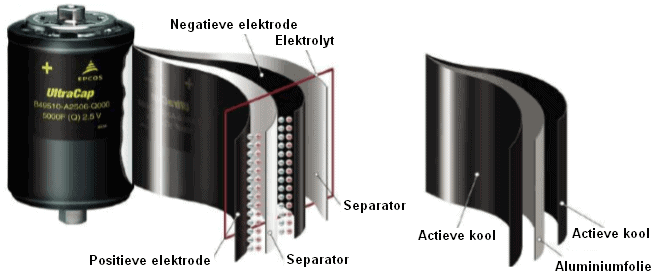
Supercaps are capacitors with a capacity and energy density thousands of times higher than standard electrolytic capacitors. The capacity is increased by using a special electrolyte (insulation material) that contains ions and therefore has a very high dielectric constant between the plates. A separator (a thin foil) is soaked in a solvent with ions and placed between the plates. The plates are usually made of carbon.
The capacity of the capacitor shown is 5000 F.
With the supercaps a combination can be made with a li-ion HV battery; during short-term acceleration, the energy from the capacitors can be used instead of the energy from the HV battery. With regenerative braking, the capacitors are fully charged within a fraction of a second. Future developments may also make it possible to replace the li-ion battery with a supercap package. Unfortunately, with current technology, the capacity and therefore the power density is too low compared to a lithium-ion battery. Scientists are looking for ways to increase capacity and power density.
Battery cell balancing:
Through passive and active battery cell balancing, each cell is monitored by the ECU to maintain a healthy battery status. This extends the life of the cells by preventing deep discharge or overcharging. Lithium-ion cells in particular must remain within strict limits. The voltage of the cells is proportional to the state of the charge. The charge of the cells should be balanced from each other as much as possible. With cell balancing it is possible to accurately control the state of charge to within 1 mV (0,001 volts).
- Passive balancing ensures an event balance in the state of charge of all battery cells by partially discharging the cells with an excessively high state of charge (we will come back to this later in the paragraph);
- Active balancing is a more complex balancing technique that can control cells individually during charging and discharging. The charging time with active balancing is shorter than with passive balancing.
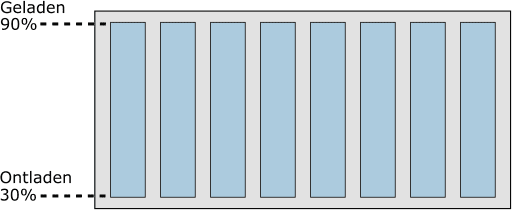
In the following image, we see an eight-cell battery module.
The eight cells are charged to 90%. The life of a cell decreases if it is continuously charged to 100%. Conversely, the life also decreases if the battery is discharged beyond 30: at a charge state of <30% the cell is deeply discharged.
The charge state of the cells will therefore always be between 30% and 90%. This is monitored by the electronics, but the driver of the vehicle does not see it.
The digital display in the dashboard shows 0% or 100% when it reaches 30% or 90%.
With age, some cells can become weaker than others. This has a major influence on the state of charge of the battery module. In the next two images we see the state of charge when two cells have a lower capacity due to age. The battery cells are not balanced in these situations.
- Discharged faster by bad cells: the two middle cells discharged faster due to their lower capacity. To prevent deep discharge, the other six cells in the module can no longer release more energy and can therefore no longer be used;
- Doesn't fully charge due to bad cells: the low capacity of the middle two cells means they charge faster. Because they have reached 90% faster than the other six cells, no further charging is possible.
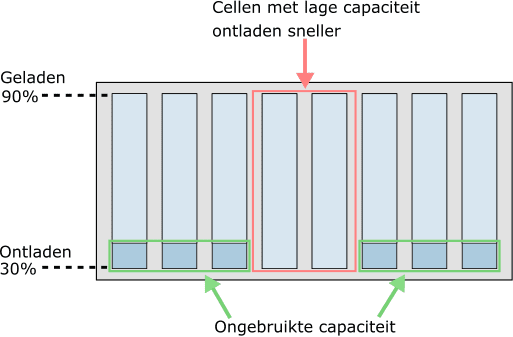
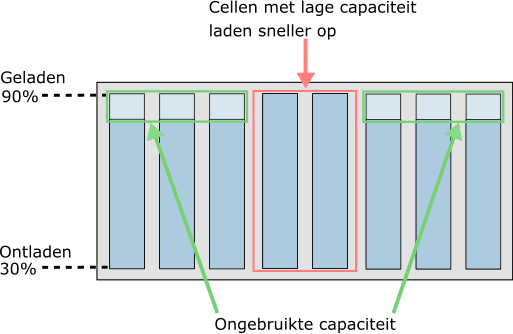
It is clear that lower capacity cells are the limiting factor in both discharging (while driving) and charging. To optimally utilize the full capacity of the battery pack and benefit for a long service life.
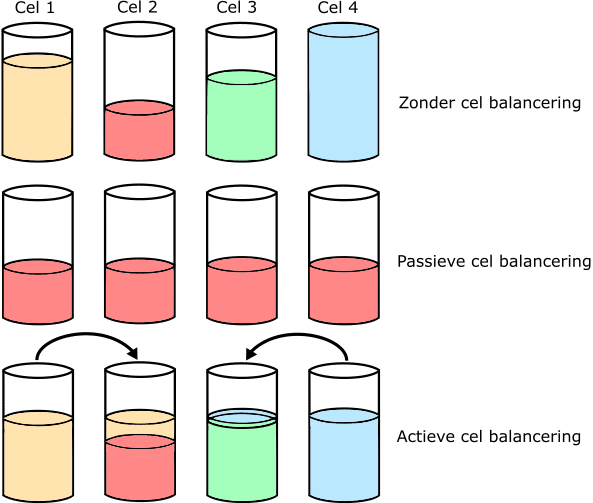
There are two methods of battery balancing: passive and active.
- Without balancing: four cells all have a different state of charge. Cell 2 is almost empty and cell 4 is fully loaded;
- Passive: the cells with the most capacity are discharged until the charge state of the weakest cell (in the example cell 2) is reached. The discharge of cells 1, 3 and 4 is loss.
In the example we see that the cups are discharged until they have reached the state of charge of cell 2; - Active: The energy from the full cells is used to fill the empty cells. There is now no loss, but the transfer of energy from one cell to another.
The working principle of the passive and active cell balancing is explained below.
Passive Cell Balancing:
In the example we see four battery cells connected in series with a switchable resistor (R) across them in parallel. In this example, the resistor is connected to ground with the switch. In reality, this is a transistor or FET.
In the example we see that cell 3 is 100% loaded. From the previous paragraphs, we know that this cell charges faster because it is weaker than the other three. Because the state of charge of cell 3 is 100%, the other three cells are no longer charged.
The resistor in parallel across cell 3 is drawn into the power circuit by the switch. Cell 3 discharges because the resistor absorbs voltage as soon as current flows through it. The discharge continues until the cell is at the level of the other cells; in this case 90%.
When all four cells in this module have the same state of charge, they can be further charged.

In passive cell balancing, energy is lost: the voltage absorbed by the parallel-connected resistors has been lost. Despite this, many manufacturers still use this method of balancing to this day.
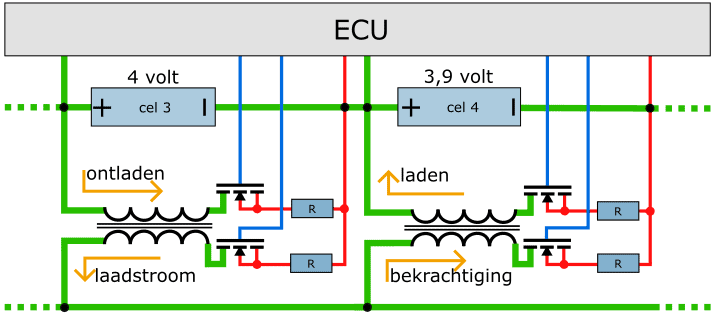
Active cell balancing:
Much more efficient is of course the active cell balancing. The energy from the overfilled cell is used to charge the empty cell. An example of the active cell balancing is shown below.
In the example we see two series connected cells (3 and 4) with their voltages (4 and 3,9 volts respectively) above them. Cell 3 is discharged by means of the transformer. The FET on the primary side allows for discharge. The primary coil in the transformer is charged with this. The FET on the secondary side turns on the secondary coil of the transformer. The resulting charging current is used to energize the transformer under another cell. The transformer below cell 4 is also turned on and off by FETs.