Subjects:
- General
- Operation
- Structure of the battery
- Plus and minus plates
- battery cells
- Charging / Discharging
- Capacity
- Cold start current
- Disconnecting the battery terminals
- Start up with jumper cables
General:
The battery has the task of supplying the energy to the consumers at times when the alternator supplies no or too little energy, for example when starting the engine. The battery is a buffer in which energy is stored. The energy provided by the alternator is stored in the battery and must be released again when it is needed. Since electrical energy is difficult to store, the electrical energy supplied by the alternator is converted into chemical energy. If the battery then has to supply electrical energy to the consumers, the chemical energy is converted back into electrical energy.
If the car battery is in good condition, but is empty again after a few hours of standing still, there may be a parasitic drain.
Operation:
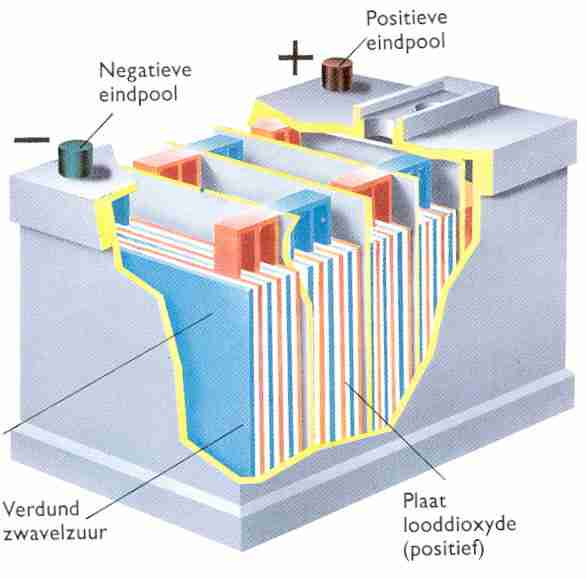
The battery contains several thin lead plates in a container with sulfuric acid. The lead bonds with sulphur. A chemical reaction then takes place. The lead is converted into lead sulphate (PbSO4).
Dilute sulfuric acid is a mixture of sulfuric acid and demineralized (purified) water. Dilute sulfuric acid is often referred to as electrolyte. When the lead plates are connected to a loading device, the lead plates will undergo a change. The plate connected to the negative releases sulfur to the electrolyte. The lead sulphate is converted to porous lead. The plate connected to the plus takes up oxygen from the electrolyte and donates sulfur to the electrolyte. This plate contains lead diocide (PbO2) after loading. The above process creates a voltage difference between the plus and minus plate.
If a consumer is connected to the lead plates that have been charged in the above-mentioned way, a current will flow. The lead dioxide of the plus plate is converted back to lead sulphate. The porous lead of the negative plate is also converted to lead sulphate. When the batteries are charged and discharged, a change occurs in the plus and minus plates (chemical effect). The electrolyte also undergoes a change during charging and discharging. When the battery is discharged, the positive and negative plates consist of lead sulphate. The sulfur used to form lead sulfate has been extracted from the electrolyte. The electrolyte of a discharged battery therefore has a low sulfur content. When the battery is charged, the lead sulphate from the plates is ceded to the electrolyte. The electrolyte then has a high sulfur content. Because the sulfur particles are the heavier particles in the electrolyte, the similar mass of the electrolyte increases as the battery's state of charge increases. The electrolyte of a fully charged battery has a similar mass of 1280 kg/m3. when the battery is completely discharged, the electrolyte has a specific mass of 1140 kg/m3. For comparison: water has a specific mass of 1000 kg/m3.
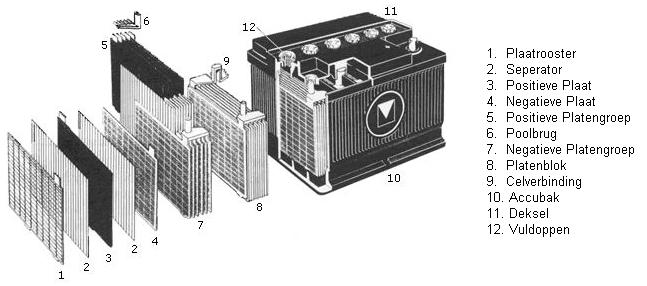
Construction of the battery:
Batteries are made up of a number of cells, each cell containing a number of plus and minus plates. Each cell has a voltage of about 2V. A 12 V battery has 6 cells connected in series. The plus and minus plates are separated from each other by separators.
Plus and minus plates:
The positive plates are connected to the positive pole, the negative plates to the negative pole. To prevent connection errors, the poles are both marked, and the positive pole always has a larger diameter than the negative pole. The plus and minus plates are connected to each other by means of a bridge piece. The plates consist of a grid of lead government. The grids are filled with paste (a mixture of lead powder, sulfuric acid and various applications). The separators are made of plastic and cellulose. During the energy conversion in the battery, more heat is developed at the plus plate than at the minus plate. To prevent warping of the plus plate, the plus plate is always placed between two negative plates.
Battery cells:
All cells of the battery are filled with the so-called electrolyte, a mixture of distilled water and sulfuric acid. Distilled (also called demineralized) water is water where contaminants such as lime and chlorine compounds have been removed. In older batteries, the cells have filling openings. Demineralized water can be topped up through these openings. The filling opening can be closed with a filling cap. Filling is no longer possible with newer batteries. They are maintenance-free batteries where the water consumption is so low that topping up is not necessary.
Charging / Discharging:
The state of charge of a battery can be measured with an acid scale. A good battery charger automatically reduces the current if the charging voltage exceeds 2,35 V per cell (so about 14 V with a 12 V battery). If this value is exceeded, the water molecules are decomposed into oxygen and hydrogen, creating hydrogen gas. If a lot of this gas is formed, it forms an explosive mixture (oxyhydrogen).
- Normal charging:
With normal charging, the capacity of the battery is brought back to 100%. The magnitude of the charging current is 5 to 10% of the capacity. A battery with a capacity of 40 Ah is charged with a charging current of 2 to 4 A during normal charging. - Fast charging: Batteries that are quickly completely discharged can be partially charged again by means of fast charging. The charging current is 30 to 50% of the battery's capacity. With a battery with a capacity of 40Ah, the charging current is 12 to 20 A. Fast charging is not often used. Many fast chargers can also be used as a jump starter and normal charger.
- Trickle charging: If a battery is not used for a long time, there will be a voltage loss due to self-discharge. By constantly connecting a trickle charger to the battery, the battery is always kept full. The charging current is approximately 0,1% of the battery's capacity. A battery with a capacity of 40 Ah is then charged with a current of 0,04 A. There are battery chargers that automatically switch to trickle charging at the end of normal charging.
- Buffer charging: With buffer charging, the consumers and the charging device are both connected to the battery. The charger supplies such a current that the battery remains practically full. The battery supplies the peak current to the users. Buffer charging takes place when the alternator charges the battery and simultaneously supplies power to the users. The alternator has a voltage regulator that is adjusted to 14,4 V for a 12 Volt installation. After starting, the alternator is fast charging for a while. While driving, the charging current drops sharply. When the battery is fully charged, the charging current becomes so small that the charger only keeps the battery charged.
If the car is in a garage, it is good to have the battery on the trickle charger. The battery then has a lower lifespan than a battery that often discharges a lot for a longer period of time and is quickly recharged by the dynamo. A battery is discharged if a consumer is left on when the engine is switched off (such as the lighting). If a battery is deeply discharged (the battery is completely empty), the battery will be damaged internally. This drastically shortens the lifespan.
Capacity:
The capacity of the battery is the maximum amount of electrical energy that the battery can hold. The capacity is expressed in Ah (ampere-hours). The capacity is determined on the basis of the test results. Example: A battery has a capacity of 60 Ah. This battery can supply a current of 20A for 3 hours. (60Ah : 20h = 3A). The terminal voltage will not fall below 1,75V per cell.
Cold Start Current:
In general, it can be assumed that the magnitude of the cold cranking current is 4 to 5 times the capacity of the battery. The cold start current provides information about the speed at which the battery can deliver electrical energy. For starter batteries used in cars, cold cranking current is even more important than capacity. The cold start current decreases sharply as the temperature decreases. This is because the chemical reactions are much slower at a lower temperature. The conditions under which the cold start current is measured are predetermined.
According to DIN standards, the following applies: the cold start current is the maximum current that the battery can supply for a certain time at a temperature of 255 K (-18 degrees) at a sufficient voltage:
- After 30 sec. Discharged with the cold start current, the terminal voltage should still be at least 1,5 V per cell.
- After 150 sec. Discharged with the cold start current, the terminal voltage must still be at least 1V per cell.
Disconnecting the battery terminals:
For certain activities (think of the airbags, starter motor, alternator) the battery must be disconnected. Otherwise, a short circuit may occur, or an airbag may be inadvertently deployed. In these cases it is sufficient to disassemble the negative terminal. The positive terminal can then remain on the battery. Never remove only the positive pole! If it touches the body (which serves as ground and is therefore connected to the negative terminal), a short circuit occurs. If the battery is removed, the negative pole must always be removed first, and only then the positive pole.
A battery should never be disconnected with the engine running. Today's engines are completely electronically controlled. The electronics can be severely damaged by the peak currents coming from the alternator.
In the past, a (non-electronically controlled) diesel engine could be disconnected in this way, because the fuel pump was mechanically driven and the injectors opened at a certain injection pressure. The mechanical operation allowed the engine to continue running without a battery after starting.
Booting with jumper cables:
If the battery is empty, the battery must be charged in order to start the engine again. This is possible to mount the battery on another car using jumper cables. It is important that good (thick) jumper cables are used. Thin cables generate a lot of resistance at high currents and therefore become very hot. There is a chance that a heavier/larger motor cannot be started with cables that are too light.
The order of connection is important; never connect the plus (red) and minus (black) cables to 1 battery at the same time, because then you can quickly have a short circuit because the contacts on the other side of the cable touch each other. Therefore, keep this order:
- Connect the negative cable to one car and the other side of the negative cable to the other car.
- Only then connect the positive cable to one car and then to the other. It doesn't matter whether the plus cable is connected first and then the negative cable, or vice versa.

Now both batteries are parallel to each other. If the batteries are parallel, the voltage will remain 12v. It is therefore not the case that the battery voltage is now 24 volts in total. That would be the case if the batteries were connected in series, which is what happens in eg electric/hybrid vehicles. For more information about series and parallel connections (using resistors as an example), see the page current, voltage resistance.
Now that the battery cables are connected, the alternator of the 'charging' car charges the empty battery. It is best to leave this for a minute, because otherwise it is possible that the engine cannot be started yet. Especially if this is a heavy diesel engine. After a minute (or longer) the car can be started with the empty battery.
The actions when disassembling the jumper cables is also important; because the car that offers the starting aid to the other car still transfers a lot of charging current via the jumper cables to the empty battery, it is not good to remove the jumper cables in one go. The charging current / voltage is very high when charging, but when disconnecting a cable, the current has nowhere to go, except in the own car electronics. There is then a current peak, which can also end up in the control units. This problem can be prevented by switching on all heavy consumers in the charging car (ie the car that recharges the empty battery). Think of the rear window heating, lighting, possibly. seat heating, etc. When disassembling a jumper cable, the peak current can be distributed in these components, which already require a lot of power. The control units are then spared. Also disassembling the jumper cables is done in the same order as connecting; first the plus or minus cable of both cars, then the other. Never remove both from 1 battery at the same time.
It is best to charge an empty battery with a battery charger, because an alternator charges it with the maximum charging current. A battery charger adapts the charging current to the condition of the battery. When a battery is deeply discharged (i.e. when the battery voltage has dropped below 6 Volt), it will be damaged internally. This drastically shortens the lifespan.
Related page:
